UPM Biomedicals produces a highly biocompatible hydrogel, GrowDex®, which enables researchers worldwide to focus on developing more human-centric tests for pharmaceutical substances, using less animal material. In order for the cells to form tiny organs (organoids and spheroids), they require a fibrous environment that mimics the extracellular matrix in our body. Typically, animal-derived materials are used to create these 3D culture conditions. GrowDex is a ready-to-use hydrogel that is extracted from birch wood and thus does not contain any material of animal or human origin.
The challenge: Dispensing viscous hydrogel with extreme accuracy using a precise valve
GrowDex is thermally stable at temperatures from 0° C to temperatures over 100° C, making it ideal for automation and high-throughput screening. GrowDex is a shear-thinning material. This means that the viscosity changes with the force exerted on the material; when the dispensing pressure is high, it is like liquid and once the pressure is removed, the material immediately sets and becomes viscous again As the nanofibers of cellulose inside the material do not cross-link, they only intertwine, the material is a true hydrogel. This means special attention needs to be paid to the pressure control generated in the dispensing system.
UPM Biomedicals therefore approached the experts at the Festo LifeTech Application Center with a request to carry out a feasibility analysis for the automated dispensing of a shear-thinning hydrogel. The aim was to reliably achieve the specified CV (coefficient of variation) values for two target volumes, 25 µl and 100 µl. These volumes represent typical volumes dispensed into 96-well and 384-well microtiter plates.
"We often dose custom liquids. When I first saw the GrowDex hydrogel in the syringes, I knew that it would not be easy to dispense it, even when it is diluted. But I also know that our system offers a lot of possibilities for finding the right solution," says Festo Application Engineer Manuel Rausch.
The approach: Varying and optimizing products and parameters
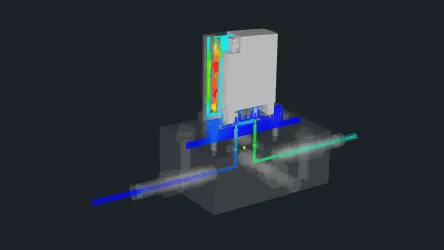
When it comes to automated liquid handling, Festo relies on the pressure-controlled dispensing method. Parameters such as pressure and opening times can be flexibly adapted to the properties of the liquid. A variety of Festo products as well as process parameters were used during the test, including media-separated valves, needles, pressures, and valve opening times.
First, the tested hydrogels GrowDex or GrowDex-T were manually diluted with the DMEM cell culture medium and transferred into a glass bottle with a lid. Pressure was added to the bottle using a pressure/vacuum generator (PGVA-1) via a connecting tube. The valve control module VAEM-V precisely controlled the media-separated valve VYKB-F12 in order to dispense the displaced hydrogel mixture into a target vessel. The dispensed quantity was recorded gravimetrically with a pair of scales and used to evaluate the CV value.
The valve VYKB-F12 and a needle with a large inner diameter proved to be the best solution to keep the flow resistance low. The chosen design also made it possible to use the compact pressure/vacuum generator PGVA-1, making a compressed air connection in the laboratory superfluous.
The outcome: The perfect dispensing process for GrowDex hydrogels
Festo Application Engineers developed a set-up consisting of components that allowed GrowDex to be dispensed even at concentrations of 0.75% and GrowDex-T at concentrations of 0.5% mixed with DMEM (1:1). The CV values were within the specified tolerance.
UPM can now be confident that its GrowDex hydrogels can be dispensed automatically in customized systems when using a specific technical set-up. "Automation can now replace another manual step, making 3D cell culture more reliable and faster," says a delighted Piia Mikkonen from UPM.
This is our joint effort to help reduce animal use, while improving the reliability and repeatability of the 3D culture results. Whether used in drug screening or in regenerative medicine, custom-built automated systems will play an important role when having to comply with regulatory requirements.
LifeTech Application Engineering – globally networked and solution-oriented
The LifeTech Application Engineering team at Festo offers customers worldwide support with their search for solutions. They carry out feasibility studies and parameter optimizations, training, prototype construction, commissioning and installations; they also clarify the requirements of customers who make a new inquiry. The application engineers have extensive experience in the areas of liquid handling, motion and liquid control.