The basic component is a rotary gripper unit that is combined with, for example, a 3D handling system. The point of departure for finding the right solution involves the dimensions of the sample vial and the required torque. Whether or not different vials will need to be handled is also decisive.
The required torque
The sample vials themselves dictate the technical requirements. What needs to be determined is how much torque is required to open and close them. The amount of required torque should always be tested in advance with the vials which will be used later on – under conditions which are as realistic as possible. If the sample vials are filled manually, the thread may be contaminated with, for example, glucose or salt-containing media. After the vial has been closed, the liquid dries out and become sticky or crystallises. Considerably higher forces are required for opening in this case. If testing is conducted exclusively with brand-new vials, there is a risk that the technical requirements are not made strict enough.
The force at the gripper can be reduced significantly if a positive-locking connection is used. On the other hand, friction-locked gripping requires much higher forces.
Torques of up to 5 Nm and gripping forces (depending on design) of up to 200 N can be implemented with standard solutions from Festo. Other torques are available on request.
The size of the vials
The necessary strokes can be determined on the basis of the height and diameter of the vials.
If the vials vary in size, a larger gripper stroke is advantageous, as is a longer Z stroke for the handling unit, so that as many requirements as possible can be fulfilled with a single system.
This greatly reduces the complexity of the overall system.
Different thread pitches
When different sample vials are handled in a single application, not only can dimensions vary greatly in some cases, but the threads in the caps may differ as well.
Simultaneous synchronisation of rotation and stroke motion during opening and closing can be very complex if it’s managed using software. A mounting adapter with Z compensation was therefore developed for rotary gripper module EHMD from Festo. The adapter automatically compensates for differences of up to 10 mm for cap thread pitch during opening and closing. Thanks to this simple mechanical solution with a passive slide, controlled motion for the Z-axis is not necessary during rotation. There’s no need to program synchronisation and the process sequence becomes significantly simpler.
Securing the vials
The vial has to be held in place while the cap is being screwed on or off in order to counteract the closing or opening torque. A clamping unit or a second gripper, for example, can be used here, since the stroke can be adapted to the different vial sizes. Depending on the vials, simple positive locking with passive clamping may also be sufficient.
Reliable closing
When the cap is closed, two threads have to be aligned. It’s possible that the cap will be placed onto the vial askew. This misalignment can result in the predefined closing torque being reached during tightening although the cap has not been correctly closed. In order to rule out this risk without expensive sensor technology, Festo has integrated a “thread engagement step” into the process. After the cap has been placed on the vial, it’s initially turned 360° anticlockwise using only slight pressure in order to align the threads on the cap and the vial. The cap is then tightened while monitoring the angle of rotation. The torque is then tested indirectly using a motor current measurement within a specified window. If no error messages are generated during this process, the vial has most probably been reliably closed.
Decapping solutions from Festo
The great number of different sample vials alone results in a wide range of technical requirements for automated opening and closing. Festo offers a large portfolio of solutions as part of its compact handling systems, from complete rotary gripper modules to solutions consisting of individual standard components to customer-specific further and new developments.
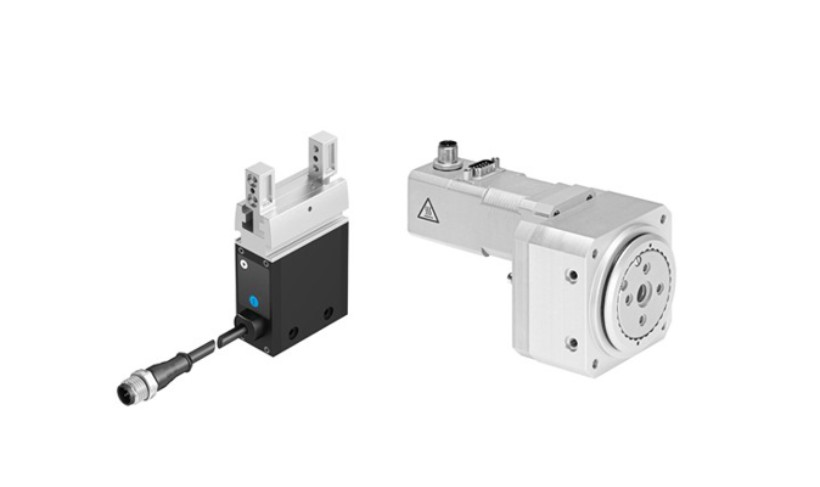
Rotary gripper module EHMD
The EHMD is the most compact rotary gripper module in its class and is especially well-suited for opening and closing small sample vials like the ones used in HPLC applications. The maximum torque value of 0.3 Nm is enough for opening vials with capacities of up to 15 ml. An optional module compensates for the cap thread pitch during rotary motion, making adaptation to a great variety of cap types and thread pitches really easy. The rotary gripper module is available in two versions: fully electric or with pneumatic gripper. Both allow for infinite rotation.

Hybrid rotary linear module DSL-16- ... -SA
The DSL-16- ... -SA is ideally suited for applications that require high torque values and gripping forces. Vials with round neck and sealing cap or cover can be gently and reliably opened with this thin, compact module with an adjustable torque of up to 1.7 Nm. The combination consisting of pneumatic stroke and gripping motion with precisely adjustable and easy-to-monitor electric rotary motion makes sample handling reliable and reproducible .

Combination of EHPS and ERMO
By combining electric standard gripper EHPS and electric rotary actuator ERMO many different requirements can be fulfilled. With the EHPS, there are three types you can choose from, with different stroke lengths for small to medium-sized vials. The ERMO is available in four sizes with maximum torques from 0.15 to 5 Nm. The rotary actuator consists of a mechanical system with permanently mounted motor and drive system (motor controller), integrated web browser technology and matching connecting cables.
Customer-specific developments
By using standardised components from Festo, a wide variety of requirements can be covered cost-effectively, with high quality and for extremely small spaces. Festo can modify existing products or develop entirely new solutions to meet very specific requests or challenges. The sooner we get involved in the planning process, the greater the advantages are for the customer. For example, Festo USA developed an extremely compact solution for sample handling that was perfectly integrated into the customer’s system, using Festo standard components and a modified rotary gripper module. This approach resulted in minimal complexity with maximum productivity and process reliability.